Product safety

Besides the recognized quality and safety, Boticário Group products are committed to offering total safety to their consumers through their products.
In our laboratories, multidisciplinary teams follow national and international standards to guarantee our cosmetics’ quality and safety. We have increased the levels of protection recommended by legislation, ensuring even more safety in the use of your cosmetics.
safety steps
Check out the steps to ensure the safety of your cosmetic:
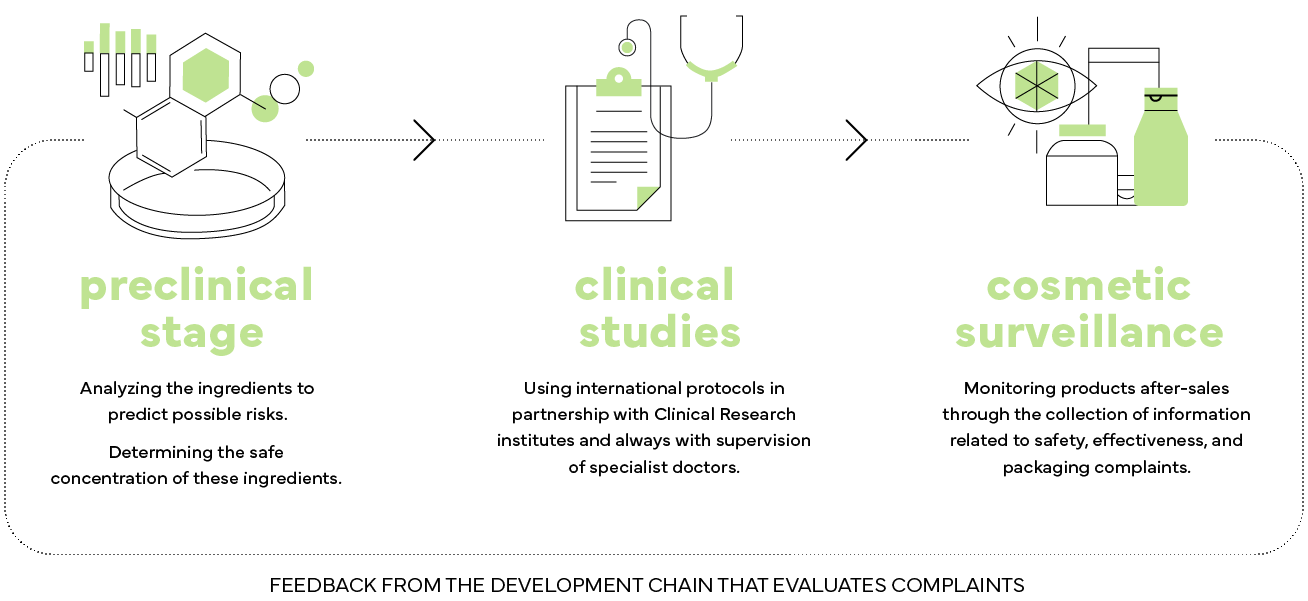
1. Preclinical
We begin our safety assessment in the preclinical phase.
In this step, scientists analyze toxicological data and chemical structure of ingredients assisted by databases and computational tools (in silico) to predict possible health risks. After this step, if necessary, in vitro studies are conducted to confirm safety and/or determine the safe concentration of ingredients by type of product and target audience.
WE START WITH:
The choice of each raw material that will be part of the formulation

A thorough toxicological analysis

Impurity limits

Definition of use concentrations
2. Clinical Studies
Product safety assurance is performed at this phase. We rely on the most advanced international protocols in partnership with clinical research institutes. In order to guarantee safety, we are always assisted by doctors, such as dermatologists, pediatricians and/or ophthalmologists. Boticário Group exports its products to several countries and, therefore, we are always aligned with international governmental and scientific bodies who issue opinions on the safety of the ingredients and whether they comply with these regulations. Therefore, you can be sure: all our products from all our own brands have gone through a rigorous and total safety assessment and can be used with maximum confidence.
3. Cosmetovigilance
Our post-sales product monitoring is performed using a system called Cosmetovigilance. Cosmetovigilance allows determining the acceptable profile of product complaints after they are introduced into the market. This collection of information and analysis of product data on the market is related to complaints about: Safety, Efficacy and Packaging. Post-sales data help us feedback the development chain that assesses whether the quantity (number of complaints) and quality (profile and severity of complaints) are within the expected for that particular product. This number and profile vary greatly according to the product and can be influenced by several factors, such as target audience, sales volume, form of use, product category, etc. Based on this information, we can further improve the performance of our products and refine future launches.
RAW MATERIALS ASSESSMENT
In Grupo Boticário, all raw materials introduced undergo a rigorous process of documentary and technical analysis to ensure they meet the group’s commitments regarding regulatory, social, environmental, and safety aspects.
TRANPARENCY AND COMPLIANCE
Detailed Documentation:
In this step, we ensure that raw materials comply with major national and international legislations. Furthermore, we verify documents that prove compliance with our non-animal testing policy and also confirm the absence of animal-derived ingredients, reaffirming our commitment to producing vegan items. In this step, we ensure that raw materials comply with major national and international legislations. A meticulous analysis of impurities present in raw materials and their safe limits is also performed, and concentration and types of use are established. Furthermore, we verify documents that prove compliance with our non-animal testing policy and also confirm the absence of animal-derived ingredients, reaffirming our commitment to producing vegan items.
Evaluation of Controversial Materials:
During the safety evaluation of raw materials, we follow a rigorous process for assessing controversial materials. These are materials currently accepted by major regulatory bodies but are somehow subject to questioning, lacking scientific consensus from a market, technical, and social perspective. Within this process, if any relevant information—through scientific investigation—indicates a risk, we include that ingredient in one of the following classifications: banned, blocked for substitution, or blocked in new products, which may impose restrictions or even prevent the entry of raw materials.
Responsibility for Human and Environmental Safety:
Our commitment to safety is fundamental and is reflected in every stage of our product development. We have a dedicated team of toxicologists who meticulously evaluate each ingredient of the raw materials. This work is based on rigorous scientific data, in silico and in vitro tests, including reconstructed skin models, to ensure the safety and effectiveness of the components. Scientific evidence is obtained through international databases and tools, or by conducting tests following globally recognized and recommended methodologies and criteria. Furthermore, we prepare complete dossiers that accompany each raw material, from its origin to its final application, ensuring transparency and compliance with the highest standards of safety and respect for the environment.
Ethical Commitment:
We guarantee the traceability of the supply chain, evaluating the social and environmental commitment of our suppliers, including fair working conditions and sustainable practices.
At Grupo Boticário, the pursuit of excellence is constant, and the care we take with our production chain is a commitment we make to our customers and to the future of our planet.
products for babies and children
The development of formulas that will be used on more sensitive skin, like babies and children’s, involves special concerns. Following our protocols, we guarantee safe and quality products.
Therefore, always keep an eye on the instructions on your product label.
Formula
The selected ingredients undergo prior evaluation by our toxicologists to ensure a safe product. We analyze the formulation predicting frequency and mode of use. We calculate the margin of safety (MoS), which is always different for children.
What is the margin of safety calculation?
It is a mathematical calculation established by international organizations that aims to guarantee the safety of substances in the concentrations applied, considering the intended use of the product and the indicated age range.
Fragrance
The choice of fragrance follows strict safety standards from the International Fragrance Association (IFRA). In addition, it follows a Fragrance Development Guide with specific premises for this public according to the age group, and it is more restricted for children up to three years old and hypoallergenic products which have restrictions on the use of substances with recognized allergenic potential. After this preclinical analysis, we move on to the final phase of the development process: our clinical tests.
Tests
These tests are conducted according to the type of product, following advanced international protocols and in partnership with clinical research institutes, always assisted by doctors, such as dermatologists, pediatricians and/or ophthalmologists. First, clinical tests are performed on adults and then are tested on children.
These steps are necessary to confirm the safety of the formula. Subsequently, we continue to closely monitor our products on the market so that we can improve their performance and refine future product launches.
All these procedures are conducted to ensure consumer safety.
sensitive skin
People with this type of skin have greater susceptibility to adverse reactions, and these reactions can be severe. Therefore, it is important to use products that are suitable for this type of skin. We know that sensitive skin has specific needs, as it has more “reactivity” to environmental factors or products such as cosmetic, and symptoms like itching, stinging, burning, among others, are manifested.

hypoallergenic
Hypoallergenic products are those whose low allergenic potential is recognized and demonstrably reduced when compared to other similar products. These products are used by consumers who are predisposed to allergic reactions, since they better accept products whose formula does not include substances that commonly cause these problems.
The choice of fragrance that will be used for these specific public also follows strict global safety standards of the International Fragrance Association (IFRA) and the Fragrance Development Guide with specific premises for sensitive skin and hypoallergenic products, which are more restricted for this public.
Our hypoallergenic and sensitive skin products are
indicated on the product labeling.
allergy, irritation and intolerances
What is allergy?
Regarding skin, allergies are reactions resulting from skin exposure to some external agent
(like ingredients contained in products) and are called contact dermatitis.
Cosmetic products can sometimes cause allergic reactions, particularly what is known as irritant contact dermatitis. These reactions are rare and usually stop when the use of the product is interrupted.
These reactions can be classified as:
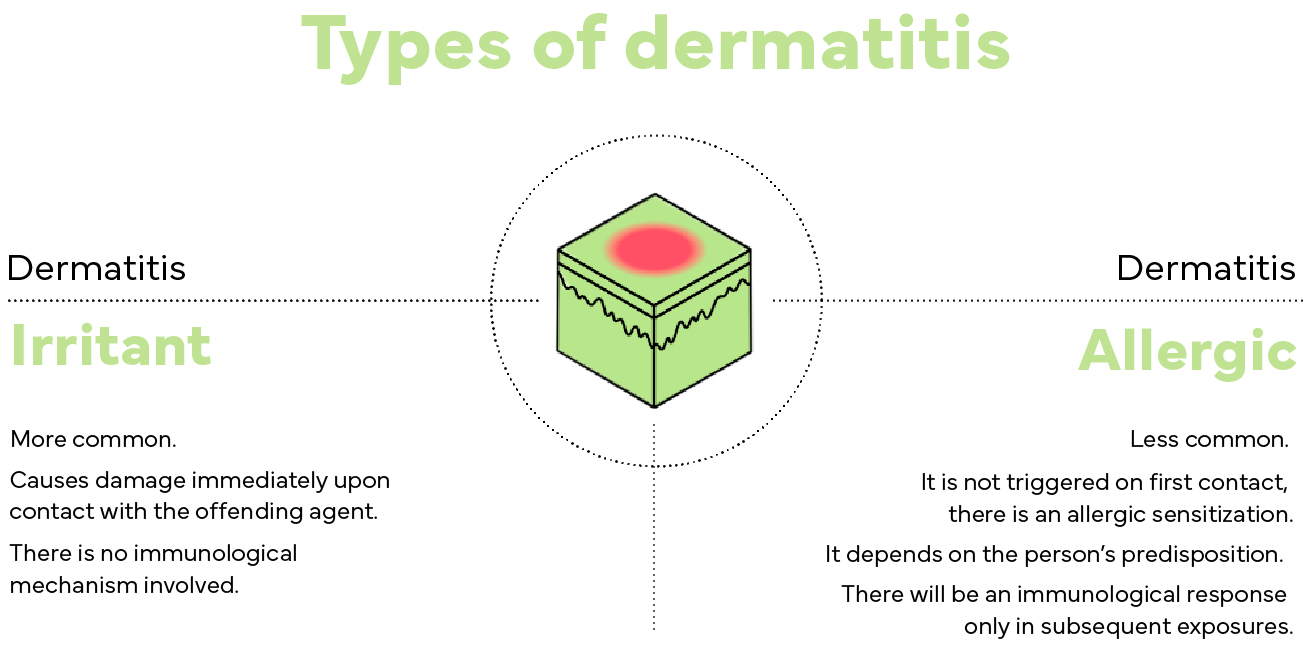
Irritant contact dermatitis
This is the most common skin allergy and is characterized by skin damage immediately after contact with the offending agent, causing an inflammatory reaction with signs of redness, itching and a burning sensation. The skin of atopic individuals (who have skin inflammation, leading to the appearance of lesions and itching) is more likely to present this type of dermatitis. There is no immunological mechanism involved.
Allergic contact dermatitis
It is less common and does not depend on the irritating action of the product on the skin, but on individual predisposition. The process is triggered throughout exposure to the product, that is, it does not appear on the first use.
allergens
Allergens are substances that are known to have a higher potential to cause allergies in sensitive individuals. They can be found naturally in the environment and in certain foods, such as dust mites, seafood, and nuts like peanuts. In cosmetics, allergens are predominantly present in fragrances, but can also be found in other raw materials, such as dyes, and in various other ingredients.
Cosmetics are subject to strict regulations, and their safety levels are extensively studied and established. Aiming at consumer transparency and safety, health authorities decided that if these substances are present in cosmetic products at a concentration exceeding 0.01% for rinse-off products and 0.001% for leave-on products, they must be indicated on the label. This indication on product labels is important to facilitate the recognition of the presence of these substances that have a higher potential to cause allergic reactions. Some examples of allergens that can be found on cosmetic labels are: citral, linalool, and limonene.
Beauty without a doubt
Unraveling Endocrine Disruptors: What You Need to Know
In recent years, there has been much discussion about substances that can mess with our hormones, called “endocrine disruptors.” They can be found in many places in our daily lives, such as plastics, cleaning products, cosmetics, food, and even water. But what exactly are they?
What are Endocrine Disruptors?
Endocrine Disruptors (EDs) are chemical substances that can interfere with our hormonal system (or endocrine system). This system is extremely important because it controls vital functions in our body, such as growth, reproductive capacity, metabolism (how our body uses energy), and even our response to stress.
These substances can be found in nature or be man-made. They act in a few ways:
- Mimicking our hormones: The body can mistake the endocrine disruptor for a natural hormone, causing it to respond incorrectly;
- Blocking our hormones: The disruptor prevents natural hormones from acting as they should;
- Altering hormone production: They can cause our body to produce hormones in excess or in insufficient amounts;
The Endocrine Society estimates that of the almost 85,000 man-made chemicals in the world, more than 1,000 may be endocrine disruptors.
The World Health Organization (WHO) defines an endocrine disruptor as “an exogenous substance or mixture that alters function(s) of the endocrine system and consequently causes adverse health effects in an intact organism, or its progeny, or (sub)populations.”
To be sure that a substance is an endocrine disruptor, scientific studies are needed to prove:
- A clear link between the substance and the hormonal alteration;
- Negative effects on the hormonal system need to be observed/proven in humans and/or animals.
It is important to know that not every substance that interacts with our hormones is an endocrine disruptor. Some may interact without causing problems, and others may even have positive effects! For example, soy contains isoflavones, which are similar to estrogen (a human hormone). Unlike endocrine disruptors, isoflavones can bring benefits in moderate amounts, such as helping to relieve menopause symptoms, protecting bones, and, for some people, reducing the risk of heart disease and breast cancer. However, these effects vary from person to person. Remember: never use medications or supplements without medical advice, even if they are natural substances!
How do we ensure the safety of our ingredients?
Here at Grupo Boticário, our priority is to create products that are safe and effective. That is why we evaluate 100% of our raw materials and products. We have a team of human and environmental safety specialists to ensure that our products are safe for everyone, including children, adults, animals, and the environment.
We follow rigorous international standards and do not use any ingredient classified as an endocrine disruptor according to:
- WHO definition;
- Classified as an endocrine disruptor according to the Endocrine disruptor assessment list of the European Chemicals Agency (ECHA);
Furthermore, we conduct computer-based analyses (called “in silico” analyses) to verify the ingredients of our products and the new molecules we develop. These tools help us identify parts of the chemical structure that might interact with hormones.
The safety of our consumers and the environment is our priority! We base everything on solid scientific evidence. There is a lot of misinformation out there, so it is crucial to always check the sources and references of what you read.On our Beleza Transparente (Transparent Beauty) page, you can find a lot of important information about our products and their ingredients. Keep an eye out because we always have updates!
